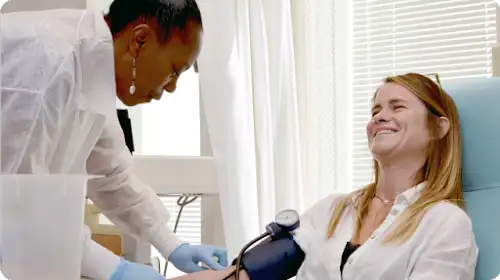
Clinical studies are closely monitored by regulatory bodies like the Food and Drug Administration (FDA), and research participants have a number of legal protections. Before they can begin, studies must receive approval from an Institutional Review Board of medical experts who review the study to ensure it’s scientifically and ethically sound. Researchers are required by law to explain the benefits and risks of participation in a way that’s simple to understand. This is called informed consent.
As a research participant, you are entitled to informed consent to any research activity you choose to join. You may choose to withdraw that consent at any time.